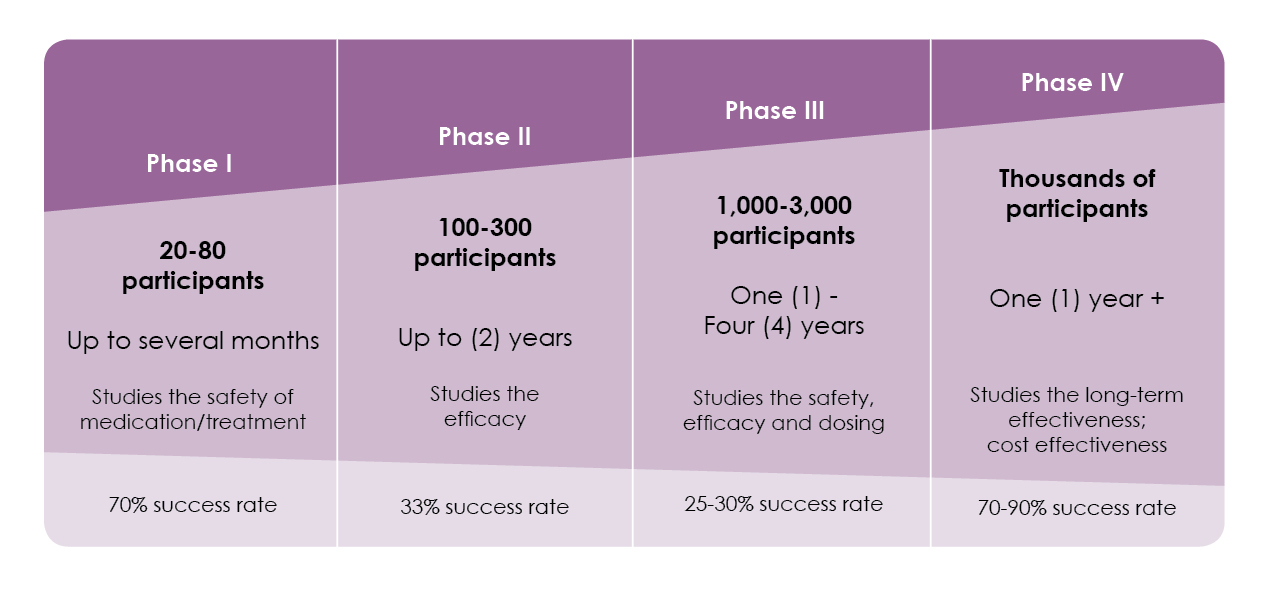
Clinical Trial Phases
Clinical trials for cancer usually follow a series of steps, called phases, which are outlined below.
Phase 1:
The first step in testing a particular medical treatment in humans. The goal is to gather information on the dosage, timing and safety of the new therapy.
Phase 2:
Designed to study the safety and effectiveness of an investigational therapy. Does the new treatment have an anticancer effect?
Phase 3:
Designed to evaluate the effectiveness of the new treatment compared to standard treatment. Is the new treatment better than current practice? Participants are assigned by chance to either an investigational group, and given the new treatment; or to a control group, and given standard treatment.
Phase 4:
Designed to further track side effects and benefits on a long-term basis after a drug has been FDA-approved.
Clinical trial phases details
When considering clinical trials for cancer, be sure to find out if some participants will be part of a control group that receives standard treatment or a placebo (an inactive substance or treatment used as a nonspecific control in a test of therapy) instead of the experimental treatment.
Many clinical trials compare a new treatment or drug with another to determine its effectiveness. Placebos would not be used in a study when the use would harm a patient or put them at risk by not providing the patient with effective treatment. Patients will be made aware if the clinical trial they are considering will use placebos in the study.
